Answer:
C. Mass defect is the energy that binds the protons and neutrons together in the nucleus.
Step-by-step explanation:
when we will find the rest mass energy of parts of nucleus i.e. proton and neutron then this rest mass energy will be more than the rest mass energy of the nucleus of atom made by same number of neutron and proton.
So here it is the difference amount of energy is considered as the energy which is used to bound all neutrons and protons together in the small space which is known as binding energy for the mass defect
here let say
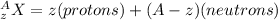
so here we will have say mass defect is given as
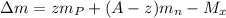
So here correct answer will be
C. Mass defect is the energy that binds the protons and neutrons together in the nucleus.