Answer:
The density of the marble is 2,000 kg/m³
Explanation:
The given parameters for finding the density of the marble are;
The volume of water in the graduated cylinder, v₁ = 20 ml
The mass of the marble dropped into the graduated cylinder, m = 8 g
The new volume of the cylinder after the 8 g marble is dropped into the cylinder, v₂ = 24 ml
Therefore, the volume of the marble, v = v₂ - v₁
Substituting the known values gives;
The volume of the marble, v = 24 ml - 20 ml = 4 ml
Density, ρ = Mass, m/(Volume, v), the units of density is kg/m³
Therefore, the density of the marble,
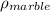 , is given as follows;
, is given as follows;
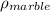 = m/v = (8 g)/(4 ml) = 2 g/ml
= m/v = (8 g)/(4 ml) = 2 g/ml
1 g/ml = 1 kg/l = 1,000 kg/m³
∴
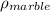 = 2 kg/l = 2,000 kg/m³.
= 2 kg/l = 2,000 kg/m³.