Answer:
8.2
Step-by-step explanation:
We have been given that the ratio of hydrogen to oxygen atoms in water molecules is 2 to 1.
We can represent this information as:
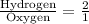
Let x be amount of moles of hydrogen atoms in water beaker. The proportion of hydrogen and oxygen moles will be same in water beaker.
Upon substituting our values in above proportion we will get,
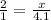
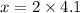
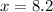
Therefore, the moles of hydrogen atoms in water beaker will be 8.2.