Answer:- 0.800 moles of the gas were collected.
Solution:- Volume, temperature and pressure is given for the gas and asks to calculate the moles of the gas.
It is an ideal gas law based problem. Ideal gas law equation is used to solve this. The equation is:
PV=nRT
Since it asks to calculate the moles that is n, so let's rearrange this for n:
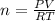
V = 19.4 L
T = 17 + 273 = 290 K
P = 746 mmHg
we need to convert the pressure from mmHg to atm and for this we divide by 760 since, 1 atm = 760 mmHg
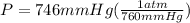
P = 0.982 atm
R =
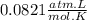
Let's plug in the values in the equation to get the moles.
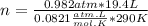
n = 0.800 moles
So, 0.800 moles of the gas were collected.