Answer: The formula of the complex will be
![[Cr(C_(10)H_8N_2)_3]^(3+)\text{ or }[Cr(bpy)_3]^(3+)](https://img.qammunity.org/2019/formulas/chemistry/college/jpojytb9aoptvwa9s2hzcfpxn1ivbq3u3m.png)
Step-by-step explanation:
Coordination number in a complex is defined as the number of ligands that are attached to the central metal atom.
- If the ligand is unidentate, it will occupy 1 place around the central metal atom.
- If the ligand is bidentate, it will occupy 2 places around the central metal atom.
- If the ligand is tridentate, it will occupy 3 places around the central metal atom.
We are given:
A metal ion having formula
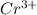 and ligand attached to it is 2,2'-bipyridine (shortly named as 'bpy')
and ligand attached to it is 2,2'-bipyridine (shortly named as 'bpy')
Coordination number of the complex is 6
We know that, 'bpy' is a bidentate ligand and does not carry any charge on it
Hence, the formula of the complex will be
![[Cr(C_(10)H_8N_2)_3]^(3+)\text{ or }[Cr(bpy)_3]^(3+)](https://img.qammunity.org/2019/formulas/chemistry/college/jpojytb9aoptvwa9s2hzcfpxn1ivbq3u3m.png)