Answer: The sign of
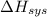 will be negative (-) and that of
will be negative (-) and that of
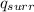 will be positive (+).
will be positive (+).
Explanation: We are given a reaction of potassium hydroxide and water which is exothermic in nature.
Exothermic reactions are the reactions in which there is release of heat energy.
- For

In these reactions, the energy of the products is lower than the energy of the reactants.
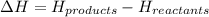
Hence, energy change for the system will be negative (-ve).
'q' is the heat.
According to the convention,
q = +ve ; when heat is absorbed.
q = -ve ; when heat is released.
As the heat is transferring from system to surrounding, so the heat is absorbed by the surrounding. Hence, the sign of 'q' will be positive (+ve)