Solution:- Hypochlorite ion
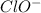 has one Cl and one O atom. Cl has 7 valence electrons and O has 6 valence electrons. Since there is one negative charge on the ion,
has one Cl and one O atom. Cl has 7 valence electrons and O has 6 valence electrons. Since there is one negative charge on the ion,
total valence electrons = 7 + 6 +1 = 14
(note:- if there is negative charge then it is added and if there is positive charge then it is subtracted while calculating the valence electrons)
Both Cl and O atoms wants to complete their octet and so for this we put a single bond between them. Single bond means two electrons, so the remaining electrons would be 14 - 2 = 12
It means 12 electrons will be placed as lone pair of electrons. To complete the octet, we put 6 dots around each of the atom. Oxygen is more electron negative than Cl, so we show the -1 charge for oxygen.