Answer :
Standard enthalpy of formation : It is defined as the enthalpy change for the reaction that forms one mole of compound from its elements. All the substances in their standard states.
The balanced chemical equation for the reaction is,
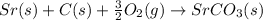
In the balance reaction, Strontium (Sr), Carbon (C) and Strontium carbonate
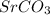 are in solid state and oxygen is in gaseous state.
are in solid state and oxygen is in gaseous state.