Answer:- c)
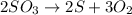
Explanations:- It asks about the equation for the formation of sulfur and oxygen by the decomposition of sulfur trioxide.
First and second equations are not correct since the are written in opposite way means sulfur and oxygen are not forming but they are reacting to form sulfur trioxide.
Fourth equation is representing the formation of sulfur and oxygen from sulfur trioxide but it is not balanced.
The third equation is the balanced equation and showing the formation of sulfur and oxygen from sulfur trioxide. So, the right choice is the third equation.