Answer:- third option is correct.
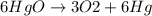
Solution:- The given equation is not balanced as the reactant side has one oxygen where as the product side has two oxygens.
As the Hg is already balanced, we need to multiply the reactant side HgO by 2 to makes two oxygen on this side. Now on doing this, Hg gets unbalanced and so to balance this we need to multiply Hg on product side by 2.
The balanced equation is:
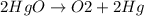
If we look at the given options then it is not one of the given choices. So, what we could do is, triple all the coefficients and see if it matches to any of the given choices.
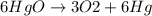
Looking at the options, the third option is correct.
 .
.