Answer:- a)
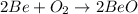
First equation is correct since Be is an alkaline earth metal and it has +2 charge. Oxygen also has -2 charge so the formula of beryllium oxide is BeO. Since, oxygen is diatomic, it is written as
 and then to balance the equation we need to multiply Be and BeO by 2 and the balanced equation is:
and then to balance the equation we need to multiply Be and BeO by 2 and the balanced equation is:
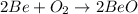
Second equation is not balanced for hydrogen and potassium. There was actually no need to multiply KCl and HCl by 3. It could simply be written as:
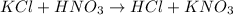
Third equation is not correct as the product formula is wrong. Mg has +2 charge and O has -2 charge, so the product will be MgO and the balanced equation must be written as:
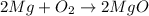
Fourth equation is not correct since metal carbonates gives metal oxide and the carbon dioxide gas on decomposition. Calcium carbonate and calcium oxide formulas are written correctly but the carbon dioxide formula is not written correctly. It must should be [tex[CO_2[/tex] where they have written
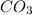 and the correct equation is written as:
and the correct equation is written as:
