Answer:- d) 42 kJ of heat is released and the reaction is exothermic.
Solution:- Heat of reaction is the summation of heats of products - reactants.
![\Delta H_r_x_n=\sum [products-reactants]](https://img.qammunity.org/2019/formulas/chemistry/college/o1fx1kfmuxsiasn3lbcymyi7wseo6wcn4q.png)
From given information, the energy contained by products is 352 kJ and the energy contained by reactants is 394 kJ. Let's plug in the values in the formula:
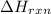 = [352 kJ - 394 kJ]
= [352 kJ - 394 kJ]
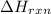 = -42 kJ
= -42 kJ
Heat of reaction is -42 kJ. The negative sign indicates the heat is released means the reaction is exothermic.
So, the correct option is the last one, 42 kJ of heat is released and the reaction is exothermic.