Answer:-
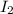
Explanations:- All these given molecules are nonpolar as the bond is between two similar atoms. The type of dispersion forces present in non polar molecules are London dispersion forces also written as LDF.
London dispersion forces becomes stronger as the molar mass if the compound increases. In all the given molecules, the molar mass of iodine is higher and for this reason the dispersion forces are also greatest in iodine.