- The answer is shorter wavelength and equal speed.
That is, compared to ultraviolet light, an electromagnetic wave that has a higher frequency will also have shorter wavelength and equal speed.
This can be seen by the reaction given below:
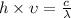
h= Planck's constant
c=speed of the light
 =frquency
=frquency
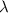 =wavelength
=wavelength
So, higher is the frequency, lesser is the volume while speed remains constant as c is speed of light.