Given the aqueous solutions of iron (II) nitrate and calcium nitrate. All nitrates are completely soluble in water.
Iron (III) nitrate ionizes into the following ions in solution:
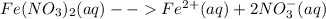
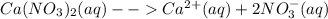
The reagent that can be used to separate the aqueous solutions of iron (II) nitrate and calcium nitrate is sulfuric acid. Calcium ions form an insoluble precipitate with sulfuric acid, where as the sulfate of iron (II) ion formed by the reaction of iron (II) nitrate with sulfuric acid is soluble.
The reaction of Calcium ion with sulfuric acid can be represented as,

White precipitate of calcium sulfate formed can be precipitated out leaving behind the solution containing iron(II) ions and other spectator ions.