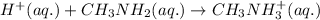Explanation: For the given acid-base reaction, The molecular equation is:
Molecular equation:
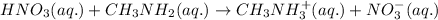
As we know,
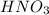 is a strong acid, so in the ionic equation, it will completely dissociate into its respective ions.
is a strong acid, so in the ionic equation, it will completely dissociate into its respective ions.
The ionic equation for the given acid-base reaction is given by:
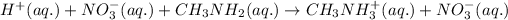
As nitrate ions are present on both the sides, so in the net ionic equation, it will get cancelled out, then the net ionic equation becomes:
Net ionic equation: