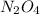Answer: Empirical formula is
 and molecular formula is
and molecular formula is
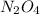
Explanation: To find the empirical and molecular formula, we follow few steps:
Step 1: Converting mass percent into mass
We are given the percentage of elements by mass. So, the total mass take will be 100 grams.
Therefore, mass of nitrogen =
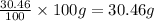
Similarly, mass of oxygen =
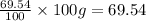
Step 2: Converting the masses into their respective moles
We use the formula:
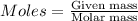
Molar mass of Nitrogen = 14 g/mol
Molar mass of oxygen = 16 g/mol
Moles of nitrogen =
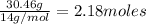
Moles of oxygen =
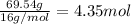
Step 3: Getting the mole ratio of nitrogen and oxygen by dividing the calculated moles by the lowest mole value.
Mole ratio of nitrogen =
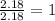
Mole ratio of oxyegn =
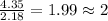
Step 4: The mole ratio of elements are represented as the subscripts in a empirical formula, we get
Empirical formula =
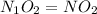
Step 5: For molecular formula, we divide the molar mass of the compound by the empirical molar mass.
Empirical molar mass of
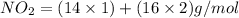
Empirical molar mass = 46 g/mol
Molar mass of the compound = 92 g/mol
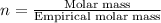
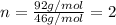
Now, multiplying each of the subscript of empirical formula by 'n', we get
Molecular formula =