Answer: 134.967 grams of Fe will be produced when 65.2 g of Al is reacted with an excess (unlimited) supply of
 .
.
Solution:
Given :
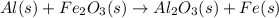
After balancing,
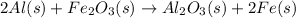
As we are given
 is in excess. Hence, Al will be considered as limiting reagent because it limits the formation of product.
is in excess. Hence, Al will be considered as limiting reagent because it limits the formation of product.
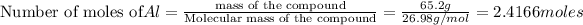
According to reaction ,2 moles of
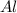 reacts with one mole of
reacts with one mole of
 to give 2 moles of Fe then 2.4166 moles of
to give 2 moles of Fe then 2.4166 moles of
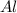 will give :
will give :
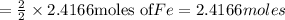
Mass of
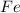 produced =
produced =
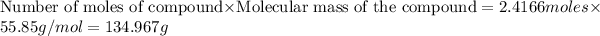
134.967 grams of Fe will be produced when 65.2 g of Al is reacted with an excess (unlimited) supply of
 .
.