Answer: 19.221 grams of oxygen gas are needed to react completely with 38.442 grams of sulfur dioxide gas.
Step-by-step explanation:
Given :
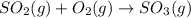
After balancing,
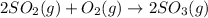
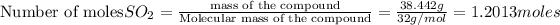
According to reaction ,2 moles of
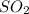 reacts with one mole of
reacts with one mole of
 then 1.2013 moles of
then 1.2013 moles of
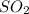 will react with :
will react with :
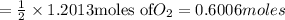
Mass of
 required =
required =
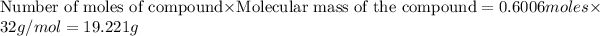
19.221 grams of oxygen gas are needed to react completely with 38.442 grams of sulfur dioxide gas.