Answer: Option (B) is the correct answer.
Step-by-step explanation:
It is known that species that dissociate in water to give hydroxide ions is known as base.
Chemical formula of milk of magnesia is
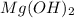 and it dissociates to give hydroxide ions when dissolved in water.
and it dissociates to give hydroxide ions when dissolved in water.
Some properties of bases are that they taste bitter, they have slippery texture, and show a pH greater than 7.
When pH of a substance ranges from 12-14 then it is known as a strong base.
And when pH is less that 7 then the substance is known as acidic in nature.
Thus, we can conclude that if the pH of milk of magnesia is between 10 and 11, it can best be described as a moderate base.