Answer:
Answer is "2"
Step-by-step explanation:
In the decomposition of potassium chlorate (KClO₃) we get KCl and oxygen gas.
Each mole of KClO₃ gives one mole of KCl and 1.5 moles of O₂ gas.
So the reaction will be:
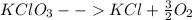
In order to convert the fractional coefficient to whole number we will multiply the complete equation with two.
![2X[KClO_(3)-->KCl+(3)/(2) O_(2)]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/y89pd8bblq5dsdiebnfceb0k1y9dxsnzsl.png)
Thus the balanced equation will be:
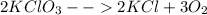
hence coefficient of KClO₃ is "2".