Answer:
chlorine-35
Step-by-step explanation:
We are given that Chlorine has an average atomic mass=35.45 amu
The two naturally occurring isotopes of chlorine are chlorine -35 and chlorine -37.
We have to find which isotope most contain 18 neutrons or 20 neutrons and why.
Let
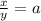
Where x= Part of chlorine-35
y=Part of chlorine-37
Then, x=a and y=1
Average atomic mass=
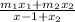
Using this formula
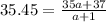
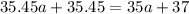
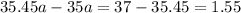
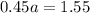
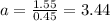
The ratio of two isotopes is 3.44 which is greater than 1.
Therefore,chlorine-35 is greater than chlorine-37 in a mixture of two isotopes.
We know that atomic number of chlorine-35 is 17.
Neutron number=35-17=18
Hence, the chlorine-35 is most which contain 18 neutrons.