So,
1. Simply count the oxygen atoms in the products.
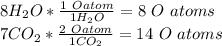
There are 22 O atoms in the products. Therefore, we should put a coefficient of 11 in front of the
 in order to have 22 O atoms in the reactants.
in order to have 22 O atoms in the reactants.
The correct answer is C.
2. Using the same conversions as in the first question, you can see that there are 2*2 = 4 H atoms in the products. Therefore, a coefficient of 2 must be placed in front of the
 to have 4 H atoms in the reactants.
to have 4 H atoms in the reactants.
The correct answer is B.
3. Check to see if there are equal numbers of each atom on both sides.
There are 2 C's on the left and 2 C's on the right. There are 4 H's on the left and 4 H's on the right. There are 6 O's on the left and 6 O's on the right (from both water and carbon dioxide). Therefore, the equation is balanced.
The correct answer is C.
4. Do the same thing as in the previous question. There is 1 Mg on both sides, but there is only 1 H and 1 Cl on the left and 2 of each on the right. Thus, the correct answer is D.