Answer:
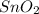 ,
,
 and
and
 are formed at the end of the reaction. They are named as tin (IV) oxide or stannic oxide, nitrogen dioxide and water respectively.
are formed at the end of the reaction. They are named as tin (IV) oxide or stannic oxide, nitrogen dioxide and water respectively.
Explanation: Reaction of tin and nitric acid is given as:
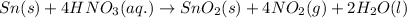
Three products are formed at the end of the reaction which are:
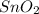 which is termed as stannic oxide or Tin (IV) oxide. This is a white colored solid.
which is termed as stannic oxide or Tin (IV) oxide. This is a white colored solid.
 which is termed as nitrogen dioxide. These are brown colored fumes.
which is termed as nitrogen dioxide. These are brown colored fumes.
 which is termed as water.
which is termed as water.
At the starting tin was a silvery-white colored solid and after the reaction, it changed the color to milky-white. This change in color is due to the chemical reaction happening between tin and nitric acid.
Release of brown fumes are also an indication that a chemical reaction has taken place.