Answer: 8 moles of ions are produced when
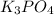 is dissolved in water.
is dissolved in water.
Explanation: When
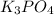 is dissolved in water, ionization reaction takes place. The reaction follows:
is dissolved in water, ionization reaction takes place. The reaction follows:
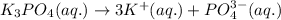
By stoichiometry,
1 mole of
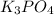 produces 3 moles of
produces 3 moles of
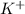 and 1 mole of
and 1 mole of
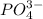
So, 2 moles of
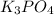 will produce (2 ×3) = 6 moles of
will produce (2 ×3) = 6 moles of
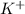 and (2 × 1) = 2 moles of
and (2 × 1) = 2 moles of
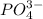
Hence, total moles of ions produced = ( 6 + 2 ) = 8 moles of ions.