Answer:
Percent by volume of ethanol = 15.5%
Step-by-step explanation:
Percent by volume is expressed as % v/v. Mathematically, it is the ratio of the volume of the solute to the volume of the solution (solute+solvent) multiplied by 100.
Formula:
%
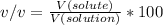
Here:
V(solute=ethanol) = 39.5 ml
V(solution = ethanol + water) = 39.5 + 215 = 254.5 ml
%
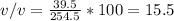 %
%