1. Equilibrium expression
Answer:
d. O₂(g)
Explanation:
2PbS(s) + 3O₂(g) + C(s) ⇌ 2Pb(s) + CO₂(g) +2SO₂(g)
The general equilibrium constant expression is
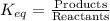
However, solids are not included in the expression because their activity (concentration) does not change.
For this reaction, the expression is
![K_(eq) = \frac{[\text{CO}_(2)][\text{SO}_(2)]^(2)}{ [\text{O}_(2)]^(3)}](https://img.qammunity.org/2019/formulas/chemistry/high-school/zicarsm2gp7js2m8sk6ru6n3lyca82gz29.png)
The only concentration term in the denominator is that of O₂(g).
2. Oxidation number
Answer:
c. +6
Explanation:
You must follow several rules to determine oxidation numbers.
The important rules for this question are:
- The oxidation number of oxygen in a compound is usually -2.
- The sum of all oxidation numbers in a neutral compound must equal zero.
Per Rule 1, the oxidation number of O is -2.
Write the oxidation number above the O in the formula:
![[\text{S}\stackrel{\hbox{-2}}{\hbox{O}}_(4)]^(2-)](https://img.qammunity.org/2019/formulas/chemistry/high-school/2cmq28ls91v5mj72h3tp2o8sidwwwilu8h.png)
There are four O atoms, so their total oxidation number is -8.
Per Rule 2, the total oxidation number of S must be +6 to make the charge on the ion -2.
There is only one S atom, so it must have an oxidation number of +6.
![[\stackrel{\hbox{+6}}{\hbox{S}}\stackrel{\hbox{-2}}{\hbox{O}}_(4)]^(2-)](https://img.qammunity.org/2019/formulas/chemistry/high-school/ei643vbm5j6jepnslgjnmwsm2ijdjjgzol.png)
3. Oxidizing agent
Answer:
b. Cl₂
Explanation:
The oxidizing agent is the substance that is reduced.
Reduction is a gain of electrons, i.e., a decrease in oxidation number.
We must identify the oxidation numbers of all atoms in the equation and discover which ones change.
I will give only the oxidation numbers that change.
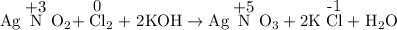
The oxidation number of N increases from +3 to +5, and that of Cl decreases from 0 to -1.
So, Cl₂ is the substance that is reduced and AgNO₂ is the substance oxidized.
Cl₂ is the oxidizing agent.