Answer:
You will obtain calcium carbonate
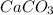
Step-by-step explanation:
First, in the combustion of a hydrocarbon you obtain carbon dioxide
 and water
and water
 . We take methane
. We take methane
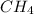 for example:
for example:
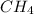 +
+
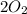 ⇒
⇒
 +
+
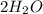
And if you pass the products to the lime water, which here it refers to a solution of calcium hydroxide
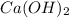 , the following reaction will take place:
, the following reaction will take place:
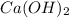 +
+
 ⇒
⇒
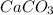 +
+

So in the final you obtain a white precipitate, which is the calcium carbonate.