Answer:
11.52 grams of sodium
Step-by-step explanation:
The balanced equation is :

As per balanced equation, two moles of sodium will give two moles of sodium chloride.
the molar mass of sodium chloride = atomic mass of Na + atomic mass of Cl
The molar mass of sodium chloride = 23 +35.5 = 58.5
thus for 58.5 grams of sodium chloride the mass of sodium required = 23 g
For one gram of sodium chloride the mass of sodium required =
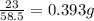
Therefore for 29.3 grams of sodium chloride = 29.3x0.393 g of sodium = 11.52g