Answer: The percent of
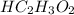 in vinegar 5.04%.
in vinegar 5.04%.
Solution:
Given :
Moles of the
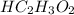 in 5 mL vinegar solution: 0.0042 moles
in 5 mL vinegar solution: 0.0042 moles
Molecular mass of the
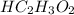 = 60.0g/mol
= 60.0g/mol
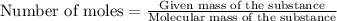
Mass of
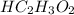 in 5 mL vinegar solution =
in 5 mL vinegar solution =
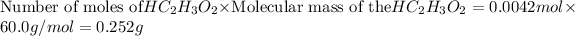
The formula to determine the percent of
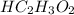 in vinegar :
in vinegar :
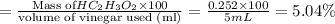
So, The percent of
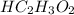 in vinegar 5.04%.
in vinegar 5.04%.