Answer:
The percent by mass of phosphorus is 22.5%.
The percent by mass of chlorine is 77.5%.
Step-by-step explanation:
Amount of compound made from phosphorus and chlorine, M = 50.51 g
Amount of phosphorus atoms were produced,x = 11.39 g
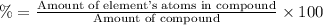
The percent by mass of phosphorus:
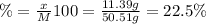
The percent by mass of chlorine:
= 100% - (percent by mass of phosphorus)%=100 % -22.5% = 77.8 %