Answer:
Kₐ = 4.06 × 10⁻⁷
Explanation:
Step 1. Calculate [H₃O⁺]
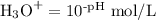
pH = 3.94
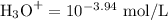
[H₃O⁺] = 1.15 × 10⁻⁴ mol·L⁻¹
Calculate
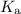
HF + H₂O ⇌ H₃O⁺ + F⁻
I/mol·L⁻¹: 0.0326 0 0
C/mol·L⁻¹: 0.0326-1.15 × 10⁻⁴ +1.15 × 10⁻⁴ +1.15 × 10⁻⁴
E/mol·L⁻¹: 0.0325 1.15 × 10⁻⁴ 1.15 × 10⁻⁴
So, at equilibrium,
[H₃O⁺] = [F⁻] = 1.15 × 10⁻⁴ mol·L⁻¹
[HF] = 0.326 – 1.15 × 10⁻⁴ mol·L⁻¹ = 0.0325 mol·L⁻¹
Kₐ = {[H₃O⁺][F⁻]}/[HF]
Kₐ = (1.15 × 10⁻⁴ × 1.15 × 10⁻⁴)/0.0325
Kₐ = 1.32 × 10⁻⁸/0.0325
Kₐ = 4.06 × 10⁻⁷
This is NOT a solution of HF (Kₐ = 7.2 × 10⁻⁴). It is more likely a solution of carbonic acid (H₂CO₃; Kₐ₁ = 4.27 × 10⁻⁷).