Answer:- A. 353.5 g
Solution:- The balanced equation is:
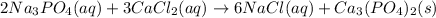
From this equation, there is 3:1 mol ratio between calcium chloride and calcium phosphate.
Grams of calcium chloride are converted to moles and these moles are multiplied by the mol ratio to get the moles of calcium phosphate. Finally the moles are multiplied by molar mass to get the grams of calcium phosphate.
Molar mass of
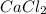 = 40.078+2(35.45) = 110.978 g per mol
= 40.078+2(35.45) = 110.978 g per mol
Molar mass of
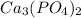 = 3(40.078)+2(30.974)+8(16.00)
= 3(40.078)+2(30.974)+8(16.00)
= 120.234+61.948+128.00 = 310.182 g per mol
let's make the set up using dimensional analysis.
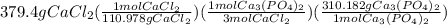
=
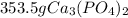
Hence, 353.5 g of calcium phosphate will form and so the correct option is A.