Answer: The option (B) is correct.
Step-by-step explanation:
According to Bronsted-Lowry acid base theory:
Bronsted-Lowry acids are those chemical species which are hydrogen ion donors
Bronsted-Lowry bases are those chemical species which are hydrogen ion acceptors.
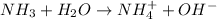
Here, ammonia is a Bronsted-Lowry base and water is a Bronsted-Lowry acid.
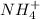 and
and
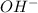 are conjugate acid and conjugate base respectively.
are conjugate acid and conjugate base respectively.
So,from the given options, option (B) is the correct answer.