We have to get the amount of CaH₂ needed to generate 48 L of H₂ gas at a pressure of 0.995 atm and a temperature of 32 °C.
The amount of CaH₂ required is 79.971 gram to generate 48 L of H₂ gas at a pressure of 0.995 atm and a temperature of 32 °C .
The number of moles of H₂ gas present in 48 litre H₂ gas at a pressure of 0.995 atm and a temperature of 32°C=
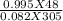 = 1.90 moles [using PV=nRT equation where, n=
= 1.90 moles [using PV=nRT equation where, n=
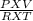 ]
]
To obtain one mole H₂, one mole of CaH₂ is required. So, to get 1.90 moles of H₂ gas, 1.90 moles of CaH₂ is required.
Molecular mass of CaH₂ is 42.09 g/mol. So, amount of CaH₂ needed to generate 1.90 moles of H₂ gas is equal to (1.90 X 42.09) gm= 79.971 gm.
79.971 grams of CaH₂ are needed to generate 48.0 L of H₂ gas at a pressure of 0.995 atm and a temperature of 32 °C.