Question 1
The correct answer is a.
Step-by-step explanation
The relationship between
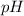 and
and
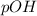 is given by
is given by
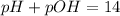 , We can use
, We can use
![pH=-log[H^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/fwbsflshee2hoks5i13wxr4q8mb2gjwctk.png) and
and
![pOH= -log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/high-school/xrzewrcngitmh86ul9i84cmycxb23ydt31.png) . In a we are given the concentration of [OH] and so we use that to find the pOH, then from pOH we can find the pH.
. In a we are given the concentration of [OH] and so we use that to find the pOH, then from pOH we can find the pH.
![pOH= -log [2.4 * 10^-^2] = 1.62\\\\pH = 14 - 1.62= 12.38](https://img.qammunity.org/2019/formulas/chemistry/high-school/7233ss3wbhnvxecuybqf8xslhkvk13imzu.png)
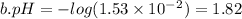
c. HCl is a strong acid so it dissociates to 0.0001
 and 0.0001
and 0.0001
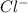 , hence
, hence
![pH= -log[0.0001] = 4](https://img.qammunity.org/2019/formulas/chemistry/high-school/ab5lr53zwyqsn7ajfcw9i1k8nkzjd4cxwa.png)
![d.pOH= -log [4.4 * 10^-9] = 8.36\\\\pH= 14- 8.36 = 5.64](https://img.qammunity.org/2019/formulas/chemistry/high-school/rqbwmepoq571v11e8kn76tt5qw8366k5e1.png)
![a.[OH^-] = 2.4 * 10^-^2](https://img.qammunity.org/2019/formulas/chemistry/high-school/7y0bvheuy81q60bx7f57dav9ulxpr3gaxy.png) has a pH 12.36 which is greater than 7
has a pH 12.36 which is greater than 7
Question 2
The correct answer is d
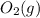 .
.
Step-by-step explanation
This is because solids and liquids do not appear in the equilibrium constant expression. Since
![K= ([products])/([reactants])](https://img.qammunity.org/2019/formulas/chemistry/high-school/8c3m6zfloia3iibnb08op9wl49nsfitjcr.png) .
.
For the equation
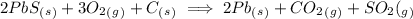
![K= ([CO_2][SO_2])/([O_2])](https://img.qammunity.org/2019/formulas/chemistry/high-school/50j7118l0xqaqsdxhlcveisi55706mj80z.png)
Question 3
The correct answer is c
In a molecule the oxidation number are assigned to get the sum of a neutral charge or ion. The overall charge of
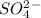 is -2 and in all its compounds oxygen has -2 charge, hence
is -2 and in all its compounds oxygen has -2 charge, hence
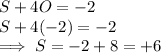
Question 4
The correct answer is b. An oxidising agent must gain electrons in a reaction, thus it is the one that undergoes reduction.In order find out what is being reduced we write half reactions for molecules that change their state.
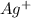 and
and
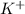 are spectactor ions in this reaction.
are spectactor ions in this reaction.
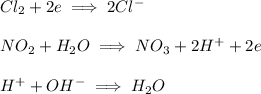
In the chloride half reaction
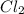 gains electrons to become
gains electrons to become
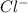 ions.
ions.
Question 5
The last element D is correct. When a nucleus decays by beta emission it produces a daughter nucleus that has same mass number but different atomic number. Therefore beta-decay will have equation
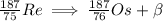
Question 6
The correct answer is a. A beta particle is an electron because it has a charge of -1 and has same mass as an electron, while a positron is a particle with the same mass as an electron but with a positive charge.