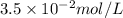Answer : The potassium ion concentration in units of mol/L is,
Explanation :
As we are given that the concentration of potassium ion in the solution is 35 meq/L. Now we have to determine the potassium ion concentration in units of mol/L.
First we have to convert meq/L to eq/L.
Conversion used :
1 meq/L = 10⁻³ eq/L
As, 1 meq/L concentration of potassium ion = 10⁻³ eq/L
So, 35 meq/L concentration of potassium ion = 35 × 10⁻³ eq/L
As we know that:
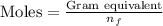
where,
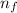 = n- factor or valency of ion
= n- factor or valency of ion
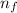 for potassium ion = 1
for potassium ion = 1
So, the concentration of potassium ion =
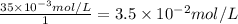
Therefore, the potassium ion concentration in units of mol/L is,