Answer: 193.3 K
Explanation:
Charles' Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
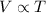 (At constant pressure and number of moles)
(At constant pressure and number of moles)
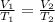
where,
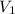 = initial volume of gas = 0.67 L
= initial volume of gas = 0.67 L
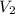 = final volume of gas =
= final volume of gas =
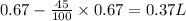
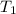 = initial temperature= 350 K
= initial temperature= 350 K
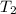 = final temperature = ?
= final temperature = ?
Now put all the given values in the above equation, we get the final pressure of gas.
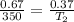
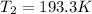
Therefore, the temperature required would be 193.3 K