8 a
The atomic symbol is
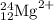 .
.
The 2+ superscript tells you that the atom has two + charges. It must add two – charges (electrons) to become a neutral atom.
The equation for the process is
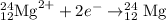
===============
8 b i
A neutral Mg atom has no charge because it has 12 protons (+)
and 12 electrons (-).
12(+)+ 12(-) = +12 – 12 = 0
===============
8 b ii
A magnesium atom loses two electrons when it forms an ion. The ion has
12 protons and 10 electrons.
12(+) + 10(-) = +12 – 10 = +2