Step-by-step explanation:
A compound is defined as a substance formed due to chemical combination of two different elements which are present in a fixed ratio by mass.
A compound is a pure substance as it can be broken down into its constituent elements.
For example,
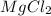 is a pure substance and it can be broken down into magnesium and chlorine ions.
is a pure substance and it can be broken down into magnesium and chlorine ions.
Thus, we can conclude that a pure substance that contains atoms of more than one element called compound.