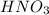Answer: The reducing agent is FeS and the oxidizing agent is
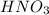
Step-by-step explanation:
Oxidizing agent is defined as the agent which oxidizes the other substance and itself gets reduced. It undergoes reduction reaction which means it gains electrons and the oxidation state of the substance gets reduced.
Reducing agent is defined as the agent which reduces the other substance and itself gets oxidized. It undergoes oxidation reaction which means it looses electrons and the oxidation state of the substance is increased.
For the given chemical equation:
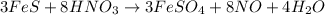
On reactant side:
Oxidation state of Iron = +2
Oxidation state of Sulfur = -2
Oxidation state of oxygen = -2
Oxidation state of hydrogen = +1
Oxidation state of nitrogen = +5
On product side:
Oxidation state of Iron = +2
Oxidation state of Sulfur = +6
Oxidation state of oxygen = -2
Oxidation state of hydrogen = +1
Oxidation state of nitrogen = +2
As, oxidation state of Sulfur is increasing from -2 to +6, it is loosing electrons and thus it is undergoing oxidation reaction and FeS is considered as a reducing agent. And the oxidation state of Nitrogen in reducing from +5 to +2, it is gaining electrons and thus it is undergoing reduction reaction and
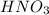 is considered as an oxidizing agent.
is considered as an oxidizing agent.
Thus, The reducing agent is FeS and the oxidizing agent is