Answer: Relative atomic mass of the element is 63.6 amu.
Step-by-step explanation: We are given an element having 2 peaks in the mass spectrum, which means that the masses of two isotopes of an element are given.
m/z value of isotope 1 or mass of isotope 1 = 63amu
m/z value of isotope 2 or mass of isotope 2 = 65amu
Fractional abundance of the isotopes can be calculated as:
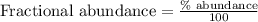
Fractional abundance of isotope 1 =
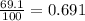
Fractional abundance of isotope 2 =

To calculate average atomic mass of an element, we use the formula:
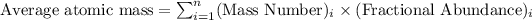
Now, putting the values of abundances and mass numbers of 2 isotopes in above equation, we get
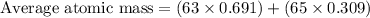
Average Atomic Mass of given element = 63.618 amu
Converting this into 3 significant figures, we get
Average atomic mass = 63.6 amu