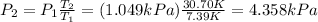Answer: 4.358 kPa
Step-by-step explanation:
The gas is contained within a rigid container, so the volume of the gas is constant. Therefore, we can use Gay-Lussac's law, which states that:
"for a gas kept at constant volume, the pressure and the absolute temperature are directly proportional"
In formulas:
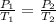
where:
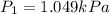 is the initial pressure
is the initial pressure
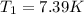 is the initial temperature
is the initial temperature
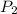 is the final pressure
is the final pressure
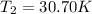 is the final temperature
is the final temperature
Substituting the numbers into the equation, we find