The final temp : 538.2 K = 265.2 °C
Further explanation
Given
Temperature : 25 + 273 = 298 K
Initial Pressure = 98 kPa
Final pressure = 177 kPa
Required
The final temp
Solution
Gay Lussac's Law
When the volume is not changed, the gas pressure is proportional to its absolute temperature
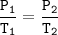
Input the value :
T₂=(P₂.T₁)/P₁
T₂=(177 x 298)/98
T₂=538.2 K