Answer: The correct statement is a single bond, because they overlap orbitals to share one pair of electrons.
Step-by-step explanation:
A covalent bond is defined as the bond which is formed when sharing of electrons between the elements forming the bond takes place. This bond is formed between two non-metals.
Fluorine is the 9th element of the periodic table having 9 electrons. The electronic configuration of this element is:
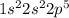
It requires 1 electron to attain stability. Thus, the molecule of fluorine will share 1 pair of electron to bond together because each fluorine atom requires only 1 electron.
Hence, the correct statement is a single bond, because they overlap orbitals to share one pair of electrons.