Answer: The number of valence electron in the given atom are 5
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in the outermost shell of an atom. Outermost shell has the highest value of 'n' that is principal quantum number.
For the given electronic configuration:
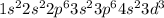
When an element belongs to d-block, the number of valence electrons are present in
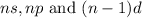 orbitals
orbitals
Here, n = 4
The electrons in '4s' orbital = 2
The electrons in '3d' orbital = 3
Number of valence electrons = 2 + 3 = 5
Hence, the number of valence electron in the given atom are 5