Answer : The volume of
 is 14.784 L.
is 14.784 L.
Solution : Given,
Mass of Aluminium = 6 g
Molar mass of Aluminium = 27 g/mole
First we have to calculate the moles of aluminium.
Moles of Al =
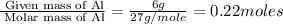
The given balanced reaction is,
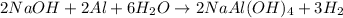
From the reaction, we conclude that
2 moles of Al react with the 6 moles of

0.22 moles of Al react with
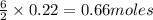 of
of

At STP, 1 mole contains 22.4 L volume
As, 1 mole of
 contains 22.4 L volume of
contains 22.4 L volume of

0.66 moles of
 contains
contains
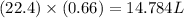 volume of
volume of

Therefore, the volume of
 is 14.784 L.
is 14.784 L.