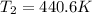Answer:
Boiling point of cyclohexane at 620 mm Hg is 440.6 K
Step-by-step explanation:
According to clausius-clapeyron equation for a liquid-vapour equilibrium-
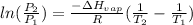
where
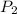 and
and
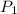 are vapor pressure of liquid at
are vapor pressure of liquid at
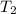 and
and
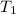 temperature respectively
temperature respectively
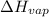 is the molar enthalpy of vaporization of liquid
is the molar enthalpy of vaporization of liquid
Let's assume
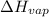 is equal to standard molar enthalpy of vaporization of a liquid
is equal to standard molar enthalpy of vaporization of a liquid
For cyclohexane, standard molar enthalpy of vaporization is 32.83 kJ/mol
Here,
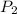 = 620 mm Hg,
= 620 mm Hg,
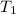 is 450.8 K (boiling point of cyclohexane) and
is 450.8 K (boiling point of cyclohexane) and
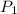 is 760 mm Hg
is 760 mm Hg
So,
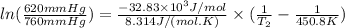
So,