Answer:- They contain the same number of atoms
Explanations:- Calcium and magnesium are two different elements and different elements have different atomic masses or molar masses. To calculate the mass we multiply the moles by the molar mass of the element. Since we have 1.0 mol of each but the molar masses are different, their masses will also be different.
1.0 mol of an element has atoms equals to Avogadro number that is
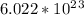 atoms. As we have 1.0 mol of each element, the number of atoms must also be same.
atoms. As we have 1.0 mol of each element, the number of atoms must also be same.
So, the only and only true statement is, "They contain the same number of atoms."