Answer : The percent composition of hydrogen in a compound is, 15%
Solution : Given,
The mass of compound = 276 grams
The mass of collected hydrogen after decomposition = 41.4 grams
Formula used :
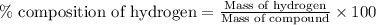
Now put all the given values in this formula, we get the percent composition of hydrogen.
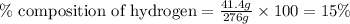
Therefore, the percent composition of hydrogen in a compound is, 15%